What is B.1.617?
B.1.617, also known as G/452.V3, is one of the known variants of SARS-CoV-2, the virus that causes COVID-19. It was first identified in Maharashtra, India on 5 October 2020. It has been referred to as a double mutation variant. "Double mutation" refers to B.1.617's mutations in the gene encoding the SARS-CoV-2 spike protein causing the substitutions E484Q and L452R. The use of the term "double mutant" has been criticized by infectious disease scientists due to SARS-CoV-2 being in a state of perpetual mutation with numerous mutations occurring around the world, so use of this misleading term is being discouraged. It is identified within the 20A clade under the Nextstrain phylogenetic classification system. With the number of cases increases in India day by day DCGI (Drug Controller General of India) nods for emergency use of drug 2-DG in India.
DCGI has approved the emergency use of a new drug developed by the Defence Research & Development Organization (DRDO) to treat the Covid-19. DRDO and Hyderabad based Dr. Reddy’s Laboratories developed the anti-covid therapeutic applications of the drug 2- Deoxy 2 Glucose (2-DG). 2-DG can be used to treat moderate to severe Covid-19 patients.
What is 2-Deoxy-D-Glucose (2-DG)?
2-Deoxy-d-glucose is a glucose molecule which has the 2-hydroxyl group replaced by hydrogen, so that it cannot undergo further glycolysis. As such; it acts to competitively inhibit the production of glucose-6-phosphate from glucose at the phosphogluco-isomerase level; thus, labeled forms of 2-deoxyglucose serve as a good marker for tissue glucose uptake and hexokinase activity. Many cancers have elevated glucose uptake and hexokinase levels. 2-Deoxyglucose labeled with tritium or carbon-14 has been a popular ligand for laboratory research in animal models, where distribution is assessed by tissue-slicing followed by autoradiography, sometimes in tandem with either conventional or electron microscopy.
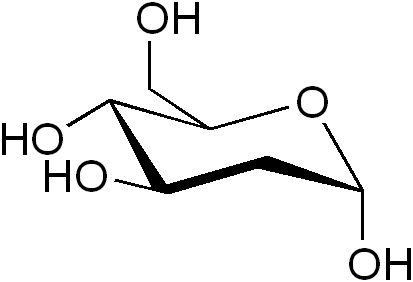
2-Deoxy-d-glucose - Image Credit - Wikipedia
Steps in India
The DRDO took initiative of developing anti-covid therapeutic application of 2-DG. In April, 2020 DRDO scientists and Hyderabad based centre for Cellular And Molecular Biology (CCMB) found that this molecule works effectively against Covid-19. Based on their work the CDSCO permitted phase II clinical trials of 2-DG on Covid-19 patients in May, 2020. And as per reports, phase – II trials between May and October 2020, the drug was found to be safe in Covid-19 patients. The treatment shows significantly improvement in the speed of patient’s recovery. The phase – II trials was conducted on 110 patients. Phase IIA was conducted in 6 hospitals and the phase IIB (dose ranging) cinicla trials was conducted at 11 hospitals across the country.
Reportedly, in efficacy trends, the patients treated with drug 2-DG showed faster asymptotic cure than Standard of Care (SoC) on various parameters and based on these results the DCGI (Drug controller General of India) permitted Phase- III clinical trials in November 2020. Phase III clinical trials were conducted on 220 patients between December 2020 and March 2021. Phase III clinical trials conducted across 27 Covid Hospitals across Delhi, Uttar Pradesh, West Bengal,Gujarat, Rajasthan, Maharashtra, Andhra Pradesh, Telangana, Karnataka and Tamil Nadu. As per phase III data, a higher proportion of patients improvedsymptomatically and was free from supplemental oxygen dependency. Similar trends were observed in patients aged over 65 years as well.
“The mechanism of action of this drug is very unique. As per the basics principle of this drug it will be very effective on different variants of SARS-CoV2-Virus. With the help of our industry partner Dr. Reddy’s Lab we are trying our best to bring this drug into the market at the earliest.” – As per a Sr. Scientist @ DRDO
Availability & Usage
2-DG comes in a power form in a sachet which is taken orally by dissolving it in water. The anti viral drug accumulates in infected cells and stops the virus from multiplying. The drug is a generic molecule and & analogue of glucose and can be easily produced and made available in plenty. Higher proportions of patients treated with the drug tested negative for covid in RT-PCR tests. Currently Dr. Reddy’s Lab is producing the drug in India.
Read also:
Can this facemask kill COVID-19 with electricity and help us to fight this pandemic?
Hydroxychloroquine And What It Does To Your Body?
References:
https://www.nature.com/articles/6600547
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6982256/
Follow us @ Facebook : Advanced Tech World





0 Comments